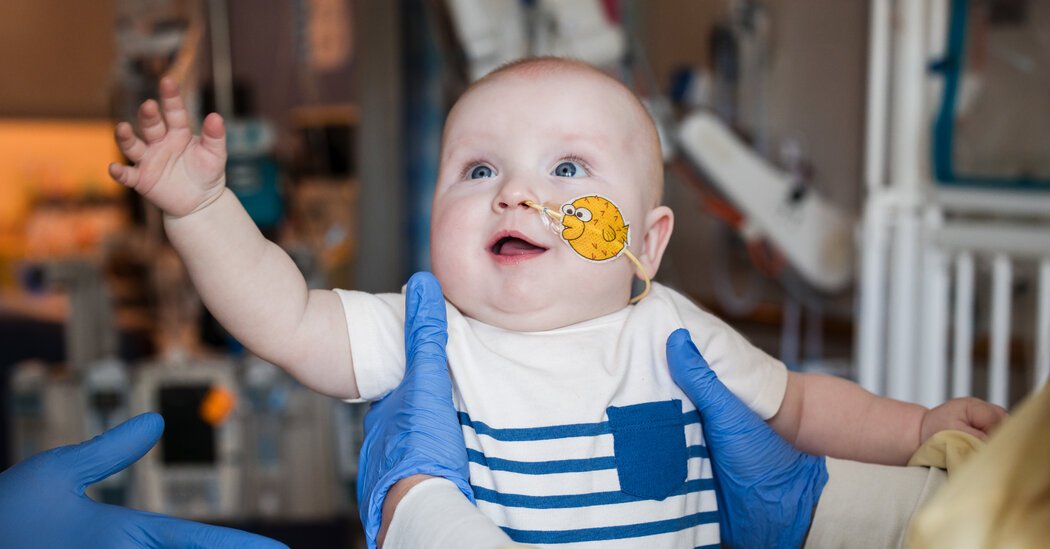
There was something very wrong with Kyle’s baby and Nicole Muldoon.
The doctors speculated. Maybe it was meningitis? Maybe sepsis?
They received an answer when KJ was only a week old. He had a rare genetic disorder, CPS1 –That only affects one in 1.3 million babies. If he survived, he would have serious mental and development delays and eventually need a liver transplant. But half of all babies with the condition die in the first week of life.
Doctors in the Children’s Hospital of Philadelphia offered the Muldoons comfortably for their baby, an opportunity to refrain from aggressive treatments in the light of a grim prognosis.
“We loved him, and we didn’t want him to suffer,” said Mrs. Muldoon. But she and her husband decided to give KJ a chance.
KJ has written medical history instead. According to his doctors, the baby, now 9 ½ months old, became the first patient of every age who had an adapted gene treatment treatment. He received an infusion that was only made for him and designed to repair his precise mutation.
The researchers who have led the effort to save KJ, present their work on Thursday during the annual meeting of the American Society of Cell & Gentapie and also publish it in the New England Journal of Medicine.
The implications of the treatment go much further than the treatment of KJ, Dr. Peter Marks, the official of the Food and Drug Administration who supervised the regulation of gene-therapy until he recently Apart from disagreements with Robert F. Kennedy Jr.The secretary of health and human services. More than 30 million people in the United States have one of the more than 7,000 rare genetic diseases. Most are so rare that no company is willing to develop a gene therapy for years that so few people would need.
But the treatment of KJ – that was built up Decades of federally funded research – Offers a new path for companies to develop personalized treatments without going through years of expensive development and testing.
Diseases such as KJs are the result of a single mutation – an incorrect DNA letter under the three billion in the human genome. Correction requires Determine targeting In an approach called Basic.
To achieve that performance, the treatment is wrapped in fat lipid molecules to protect it against breakdown in the blood on the way to the liver, where the operation will be made. In the lipids there are instructions that the cells recommend to produce an enzyme that processes the gene. They also wear a molecular GPS – CRISPR – that was changed to crawl past a person’s DNA until it finds the exact DNA letter that must be changed.
Although KJ’s treatment was adjusted, so CRISPR only found its mutation, the same type of method could be adjusted and always used to repair mutations in other places on the DNA of a person. Only the CRISPR instructions that lead the editor to the location on the DNA with the mutation should be changed. Treatments would be cheaper, “at least with an order of size,” said Dr. Marks.
The method, Dr. said Marks, who wrote An editorial In the research paper, “is one of the most potential transformational technologies that exist.”
It can ultimately also be used for more usual genetic disorders such as sickle cell disease, cystic fibrosis, Huntington’s disease and muscular dystrophy.
And, he said, “it could really transform health care.”
The story of the customized treatment of KJ started on the evening of 8 August, then Dr. Kiran Musunuru, a gene-working researcher at the University of Pennsylvania, received an e-mail from Dr. Rebecca Ahrens-Nicklas in the children’s hospital of Philadelphia. A baby was born and genetic testing showed that he had CPS1 deficiency.
Can he save the baby?
Dr. Musunuru had started investigating the use of gene processing for fairly common gene mutations.
Developing a Geneditor to treat patients is a deliberate process that can last for years. But KJ did not have years to wait – perhaps as little as six months before a risk of serious brain damage or death at the time.
“At this point the clock starts in my mind,” said Dr. Musunuru. “This is real life. This is not hypothetical.”
KJ’s disease is caused by an inability to rid the body of ammonia, a by -product of protein metabolism. Ammoniak builds up in the blood and crosses the brain. His doctors put him on a diet that protein is seriously limited – just enough for him to grow. He also had a medicine, glycerol phenylbutyraat, that helped to remove the ammonia in his blood. But he still ran a high risk of brain injury or death. Every disease or infection can increase its ammonia levels and cause irreversible damage to his brain.
KJ lived in the hospital under 24 hours of care.
Building a gene processing system for the baby of the Muldoons and testing it was not easy.
“There was a lot of shooting from the hip,” said Dr. Musunuru.
He started working with Fyodor Urnov at the University of California, Berkeley, who ensured that there were no unexpected and harmful gene operations elsewhere in the DNA. Dr. Urnov is part of an academic collaboration with Danaher Corporation, a company that is able to produce the Geditor for KJ with a standard that could use it with a patient.
Danaher, in turn, collaborated with two other companies that owned it, two extra biotechnology companies and another research institute, said Sadik Kassim, his Chief Technology Officer for genomic medicines.
“At every step of the process we always expected someone to say,” No, sorry, “said Dr. Kassim.” And that would be the end of the story. “But his fears were unfounded. Danaher and the other companies only accused the raw materials to make the medicine, he added.
The FDA has also smoothed out the approval of treatment regulations, Dr. Ahrens-Nicklas.
Dozens of researchers have already set aside the other for months.
In Berkeley, Dr. Urnov: “Scientists burned a barrel of midnight oil at this size of San Francisco Bay.” He added that “such a speed for producing a crispr of clinic quality for a genetic illness has no precedent in our field. Not even close.”
David Liu van Harvard, whose Lab has invented the gene adaptation that was used to repair the mutation of KJ, said that the speed was ‘amazing’.
“These steps traditionally take most of a decade, if not any longer,” he said.
Only when the solution for editing genes was in control and the FDA approved the work of the researchers, Dr. Ahrens-Nicklas the parents of KJ.
“One of the most frightening moments was when I walked into the room and said:” I don’t know if it will work, but I promise I will do everything to make sure it is safe, “she said.
On the morning of 25 February, KJ received the first infusion, a very low dose because nobody knew how the baby would react. He was in his room, in the cradle where he had lived all his life. He was 6 months old and in the seventh percentile for his weight.
Dr. Musunuru followed the two -hour infusion, the feeling, he said, “both excited and terrified.”
KJ slept through it.
Within two weeks, KJ was able to eat as many proteins as a healthy baby. But he still needed the medicine to remove the ammonia from his blood – a sign that the Geneditor had not yet corrected the DNA in every affected cell.
The doctors gave him a second dose 22 days later.
They were able to halve the medication dose. At that time he received a few viral diseases, which would normally have caused terrifying streets in his ammonia levels. But, Dr. Ahrens-Nicklas said, “He sailed through it.”
A week and a half ago the KJ team gave a third dose.
It is too early to know if he can stop taking the medicine completely, but the dosage has been greatly reduced. And he is good enough for the team to plan to dismiss him home from the hospital. He meets developmental mile poles and his weight is now in the 40th percentile for his age, but it is not yet known whether he will be saved a liver transplant.
The result “is a triumph for the investment of American people in biomedical research,” said Dr. Urnov.
The researchers emphasized the role of government financing that played in the development.
The work, they said, started federal financing for basic research into bacterial immune systems decades ago. That ultimately led to the discovery of CRISPR. Federal investments in sequencing the human genome made it possible to identify the mutation of KJ. American financing supported Dr. Lab. Liu and the editing. A federal program to study gene processing supported Dr. Musunuru. Going parallel, was federally financed work that led to a concept of KJ’s disease.
“I don’t think this could have happened in a country other than the US,” said Dr. Urnov.
Those who were saving KJ were proud, Dr. Urnov.
“We all said to each other:” This is the most important thing we have ever done. “
- Advertisement -