Breakthrough vaccine that virtually eliminates painful eczema gets green light from NHS
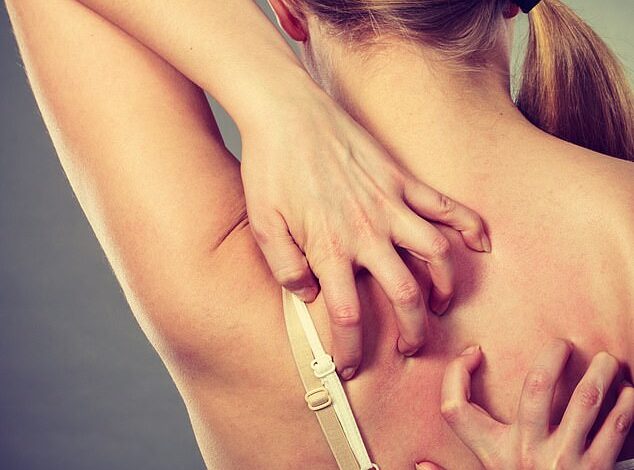



Thousands of people with eczema could soon see their painful symptoms disappear as the NHS announces the introduction of a groundbreaking new vaccine.
The monthly injection can be used by adults and children aged 12 years and over who suffer from the inflammatory disease, which causes annoying patches of itchy, cracked and dry skin.
Lebrikizumab, which was approved by EU regulators last year, has been shown to completely clear eczema in four out of five people given the drug. It eases symptoms by targeting a protein in the body that causes inflammation.
And now the groundbreaking treatment is available on prescription in England and Wales after the NHS spending regulator gave it the green light.
For Kymmene Dawson, 39, starting lebrikizumab has been a life-changing experience. “It’s given me a new lease on life, where I wake up in the morning and know I can go about my day without feeling insecure or having to cancel plans,” she said.

Thousands of eczema sufferers could soon see their painful symptoms disappear (stock image)

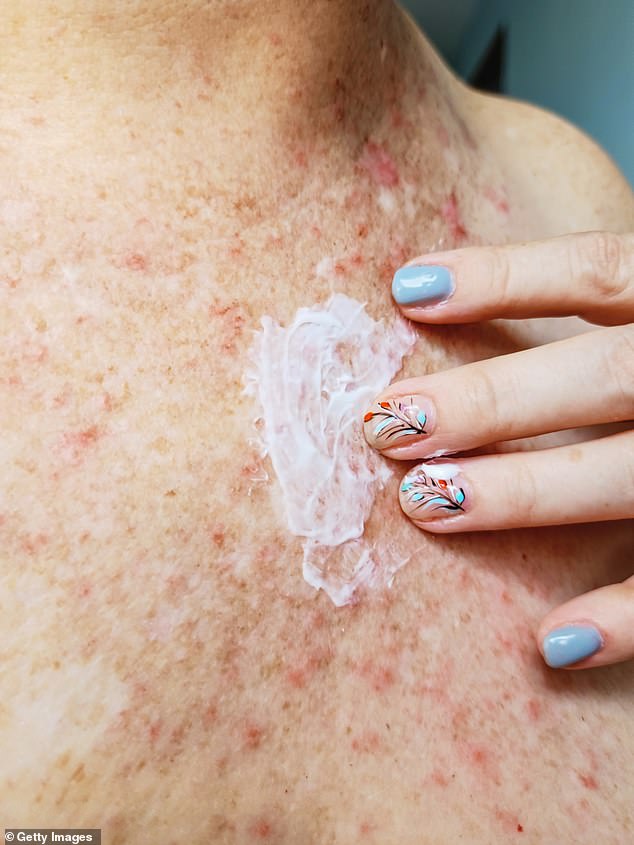
The monthly injection can be used by adults and children over 12 years of age who suffer from the inflammatory condition, which causes uncomfortable patches of itchy, cracked and dry skin (eczema stock image)

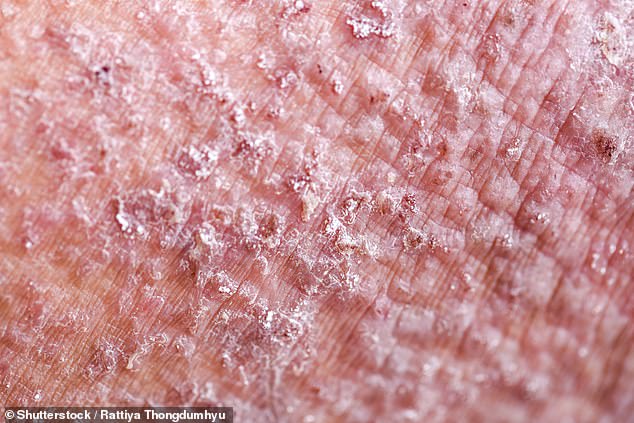
Pictured: A close-up of atopic dermatitis, also known as atopic eczema, one of the most common forms of eczema
Kymmene, who runs a beer bar in Salford called Joule, was offered the vaccine in March after previous vaccinations failed to treat her severe eczema, which mainly affected her face.
“I’ve had eczema my whole life, but for the last 10 years I’ve needed hospital treatment and immunosuppressive drugs,” she said. “Last summer I was housebound because my skin was so bad. I’d tried everything and it was bad.”
In 2021, she was prescribed dupilumab, which initially seemed to transform her skin as well. But after six months, she started experiencing very severe flare-ups again.
However, within the first month of taking lebrikizumab, Kymmene saw her skin start to clear up and her itching subside. “It had a positive effect very quickly and now I’m in the best shape I’ve been in for a while,” she said. “I don’t feel like I have eczema anymore – that’s a pretty big deal.”
Kymmene did experience some side effects, including small cuts on the inner and outer corners of her eyes, but said these largely disappeared and her eyes felt better than ever.
She also experienced mild joint pain for a few days after receiving the lebrikizumab shot.

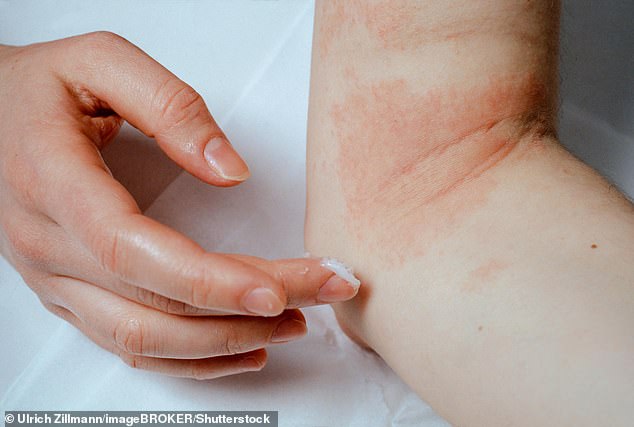
Pictured: A woman applying ointment to eczema on her elbow
“Overall I am very happy with everything and I plan to continue taking the medication as long as it works,” she added.
Andrew Proctor, chief executive of the National Eczema Society, welcomed the news that lebrikizumab would be available on the NHS, saying: ‘Most people have heard of atopic eczema but don’t realise how it can dominate the lives of patients and their families. Living with more severe atopic eczema requires constant planning and preparation.
‘Besides a time-consuming and messy skin care regimen and regularly disrupted sleep, living with eczema can be an all-consuming chore for patients and parents of children with the condition.
“It’s important that we have a range of treatment options so that patients have the opportunity to get a treatment that’s right for them.”
Over 5.2 million adults and 2.5 million children in the UK currently suffer from moderate to severe cases of eczema. The condition is usually treated initially with prescription washes and steroid creams to reduce swelling and redness. In more severe cases, immunosuppressive drugs are given, which can have a negative effect on immunity and the liver.
The revolutionary new drug dupilumab was approved by the NHS in 2021 for patients who have not responded to other treatments.

The revolutionary new drug dupilumab (pictured) was approved by the NHS in 2021 for patients who have not responded to other treatments

Professor Tony Bewley (pictured), a dermatologist at Bart’s Health NHS Trust in London, said the rollout will be ‘very welcome for both patients and clinicians’
The shot targets two proteins found in the immune system – interleukin-4 and interleukin-13 – which cause inflammation. The shot has been shown to be effective in reducing the symptoms of eczema.
However, it can also cause side effects such as conjunctivitis – where the eyes become red and painful – and joint pain. Trials of lebrikizumab, which targets only the interleukin-13 protein, showed that the drug caused far fewer side effects than dupilumab.
Research also found that 80 percent of participants with moderate to severe eczema who took lebrikizumab for two years maintained clear or almost clear skin. Experts say this could help people who stop taking the drug live eczema-free.
Earlier this month, the National Institute for Health and Care Excellence, which decides whether the NHS will fund new treatments, concluded that lebrikizumab can be offered to patients who have already responded to an immunosuppressant or where these drugs are not considered suitable.
Professor Tony Bewley, a dermatologist at Bart’s Health NHS Trust in London, said the rollout will be “very welcome for both patients and clinicians”.
“Atopic dermatitis has a psychosocial burden for patients and their families that is often underestimated,” he said.
Both adults and children often face stigma, loss of self-confidence, bullying, sleep deprivation and overwhelming itching, which can affect their performance at school and work.
‘In severe atopic dermatitis, patients often suffer from depression, anxiety and even suicidal thoughts. The addition of this therapy is an important step forward and very welcome for both patients and clinicians.’




