Revealed: The bowel cancer breakthrough that could save young patients — and the signs doctors may miss



A bowel cancer treatment that offers hope to people with the same aggressive form as Dame Deborah James has has been approved for use by the NHS.
The drug combination – two drugs called trifluridine-tipiracil hydrochloride and bevacizumab – will be launched after a US study found it could add almost an extra year to patients who had run out of treatment options.
In rare cases, patients could even last seven years longer.
According to experts, it is an important step in the fight against the BRAF V6004E mutation, an incurable genetic form of colon cancer that often affects young people.

Dame Deborah James had the aggressive BRAF mutation. Under the name Bowelbabe, the deputy director built up a huge following on social media

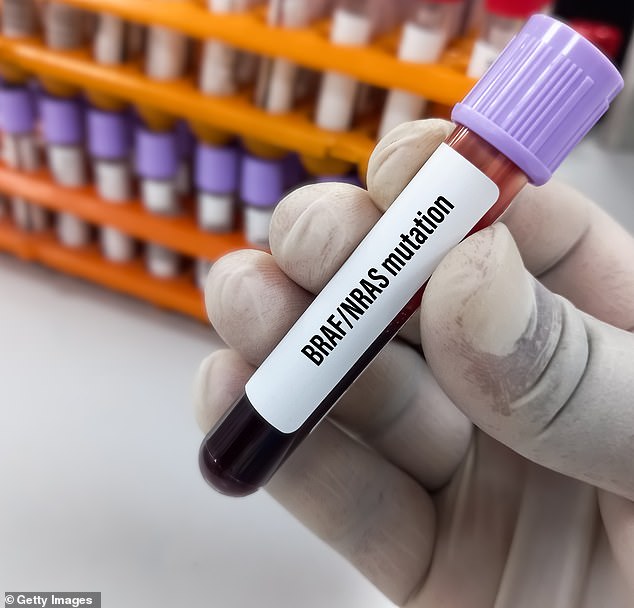
A blood sample taken as part of testing for the mutation. Most patients do not survive longer than ten months after diagnosis
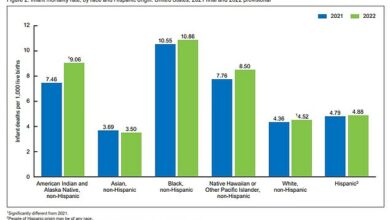
Of the 42,000 Britons diagnosed with bowel cancer each year, around one in ten carry the BRAF mutation. Chemotherapy and other drugs quickly become ineffective and most patients do not survive for more than ten months after diagnosis.
Dame Deborah was just 35 in 2016 when she was diagnosed with BRAF bowel cancer. Under the name Bowelbabe, the deputy director built a huge following on social media, documenting her progress and raising awareness of the disease and its symptoms until her death in 2022.
The drug combination has been available privately in the UK for eight years and costs around £2,000 a month.
However, last month the NHS spending watchdog, the National Institute for Health and Care Excellence (NICE), gave the green light for patients who have already had two failed treatments, in a move predicted to benefit around 1,000 people a year.
“Many patients have been on this regime for years, which stops the cancer developing,” says Anna Bennett of the patient group Breaking BRAF, which campaigns for new treatments.
“Some have achieved stability for over a year or two and we know of one patient who has lived almost seven years longer. We are pleased with this decision.”
Professor Marco Gerlinger, head of the department of gastrointestinal cancer medicine at Barts Cancer Institute in London, called the approval of the drug combination a “fantastic development”.
“For the past decade, there have been no highly effective drugs for this type of cancer,” he adds. “But now we have a really great option for patients.”
The BRAF mutation causes cancers to grow faster than they otherwise would. For this reason, people who carry it often show symptoms at a young age.
The mutation is also linked to skin and brain cancer and appears to be more common in women.
BRAF cancers quickly become resistant to treatment, meaning patients need multiple treatments to stay alive.
During her six-year battle with bowel cancer, Dame Deborah underwent more than a dozen operations, 100 chemotherapy treatments and more than ten targeted drug treatments.
Trifluridine-tipiracil hydrochloride – also known as Lonsurf – is a daily tablet that stops new cancer cells from growing. Bevacizumab, also known as Avastin, works by blocking the formation of new blood vessels that feed tumours and help them grow. It is given intravenously in hospital every two weeks.
Both drugs are used as long as they are effective.
The clinical trial, reported last year in the New England Journal of Medicine, found that the combination gave an average of 10 months of extra survival, but many patients lived up to a year and a half longer. The drugs also caused few serious side effects, the most common of which were nausea and vomiting.
While patient groups welcomed NICE’s decision to fund the treatment, they criticised the delay, which left many desperate patients turning to private clinics.
“It is inexcusable that we have ended up in a situation where so many patients have died without access to a drug that could have given them months, if not years, of extra life,” said Ms Bennett, whose sister Rebecca died of bowel cancer,

Mother of two Emily Georghiou, 47, from London, was diagnosed with bowel cancer in 2021
‘People now have hope, whereas before they faced the terrible choice between life or debt.’
One patient who could benefit from the combination is Emily Georghiou, a mother of two from London, who was diagnosed in 2021.
“Ever since chemotherapy failed for me and I found out I have the aggressive BRAF mutation, I’ve felt like a ticking time bomb,” says Emily, a former health policy adviser. “We don’t have the money or private insurance to pay for medication, and even though I started a fundraiser, I knew I would eventually run out of money to stay alive.
‘If you have this condition, you live from scan to scan. At any time, your condition can change.
‘This announcement gives me hope because when that time comes, I now know it will be there.
‘It is extremely important that I can be with my beautiful boys for as long as possible.’
The patient group Breaking BRAF hopes that another promising drug combination will soon be approved:botensilimab and balstilimab. The group also states that it is crucial that GPs are reminded of the signs of bowel cancer in young people, such as blood in the stool, unexpected weight loss, fatigue or the feeling that the bowels are not completely empty.
‘So many patients, including my sister, have been turned away by GPs until it becomes an emergency,’ says Ms Bennett. ‘With bowel cancer becoming increasingly common in young people, it is crucial that doctors are aware of the early symptoms.’